
Motiva® Safety Through Innovation
With over 3 million implants sold, spanning more than 12 years, in 85 countries worldwide,
Motiva Implants® have consistently reported superior safety outcomes. This includes rates of less than 1% of device-related complications that lead to reoperation, such as capsular contracture and implant rupture.
The low rates of capsular contracture with Motiva Implants® are consistent across all surgical planes: submuscular, subglandular, or subfascial. The worldwide rate of reoperation due to rupture with Motiva Implants® is lower than 0.1%.
Preliminary clinical results from the Motiva® IDE study in the United States, which is still in its follow-up phase, are encouraging and with a high rate of patient follow-up. The 3-year Kaplan- Meier risk rate of occurrence for capsular contracture and implant rupture are lower than 1%.
The strong safety and performance of Motiva Implants® are confirmed by international registry data and independent peer-reviewed publications from around the world.
Post-Market Surveillance
3,000,000-plus:implants sold globally since commercial launch in 2010
85-plus countries:where Motiva® Implants have been approved
85-plus publications:Evidence-based research supporting the science, engineering, and clinical outcomes with Motiva Implants®
Less than 1%:consistently low device-related complications
EVOLUTION OF BREAST IMPLANTS
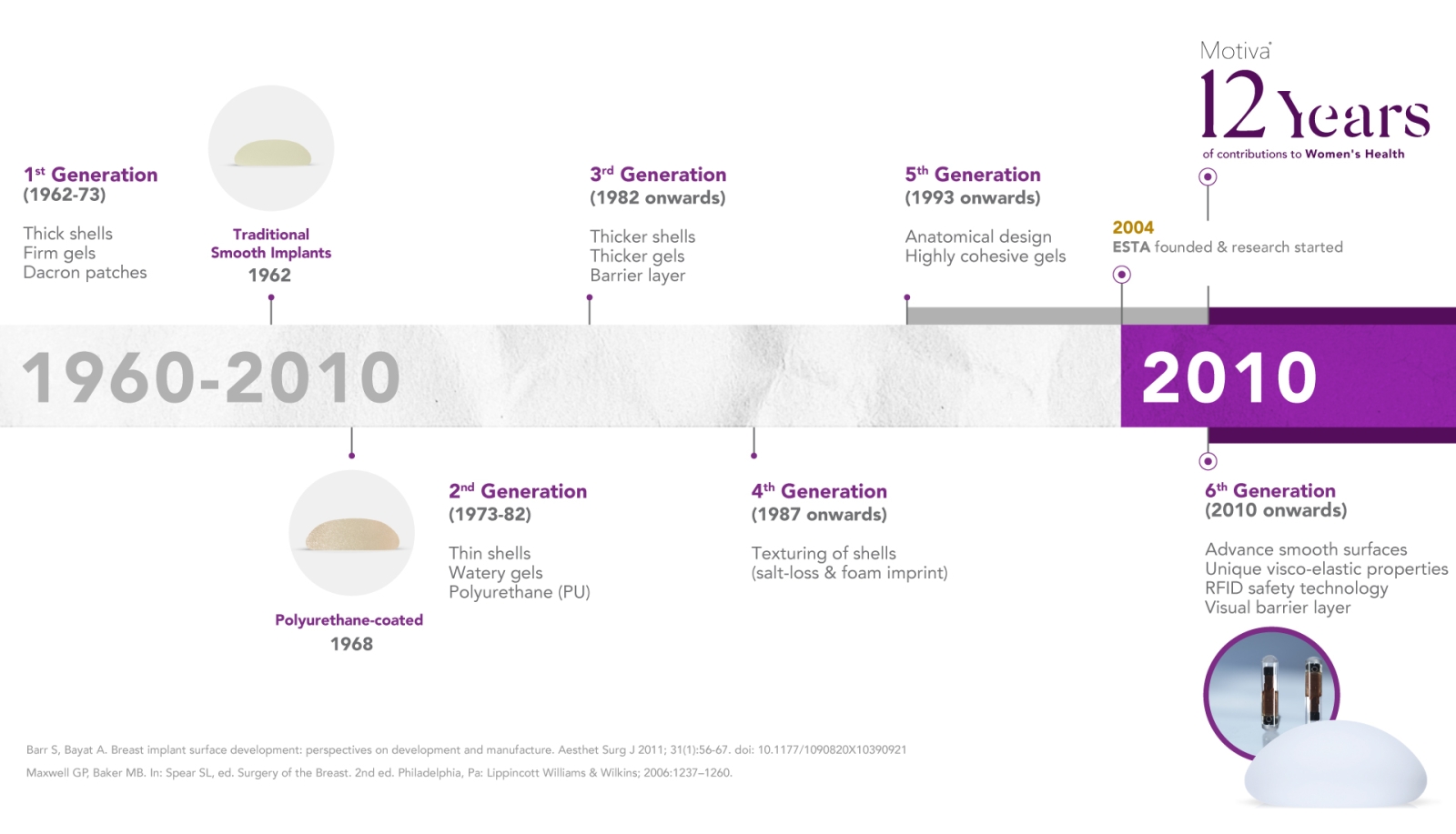

Motiva® 6TH Generation Breast Implants
SMOOTHSILK®/SILKSURFACE® IS DESIGNED TO:
- Minimize inflammation and improve interaction with your body's tissues (biocompatibility)
- Enable easier implant insertion for minimal scarring
- Limit the risk of double capsules and late seromas
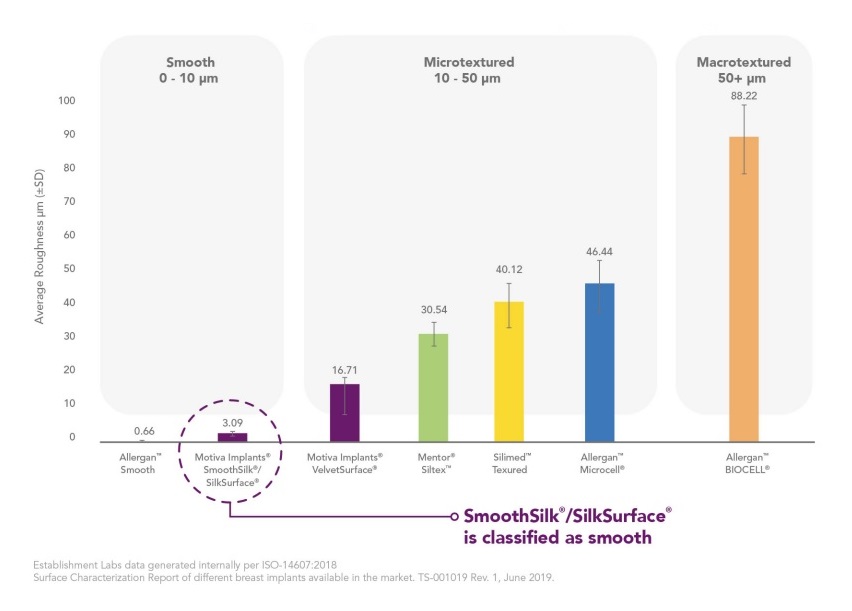
Breast Implants classification according to its surface
International Organization for Standardization (ISO-14607:2018)
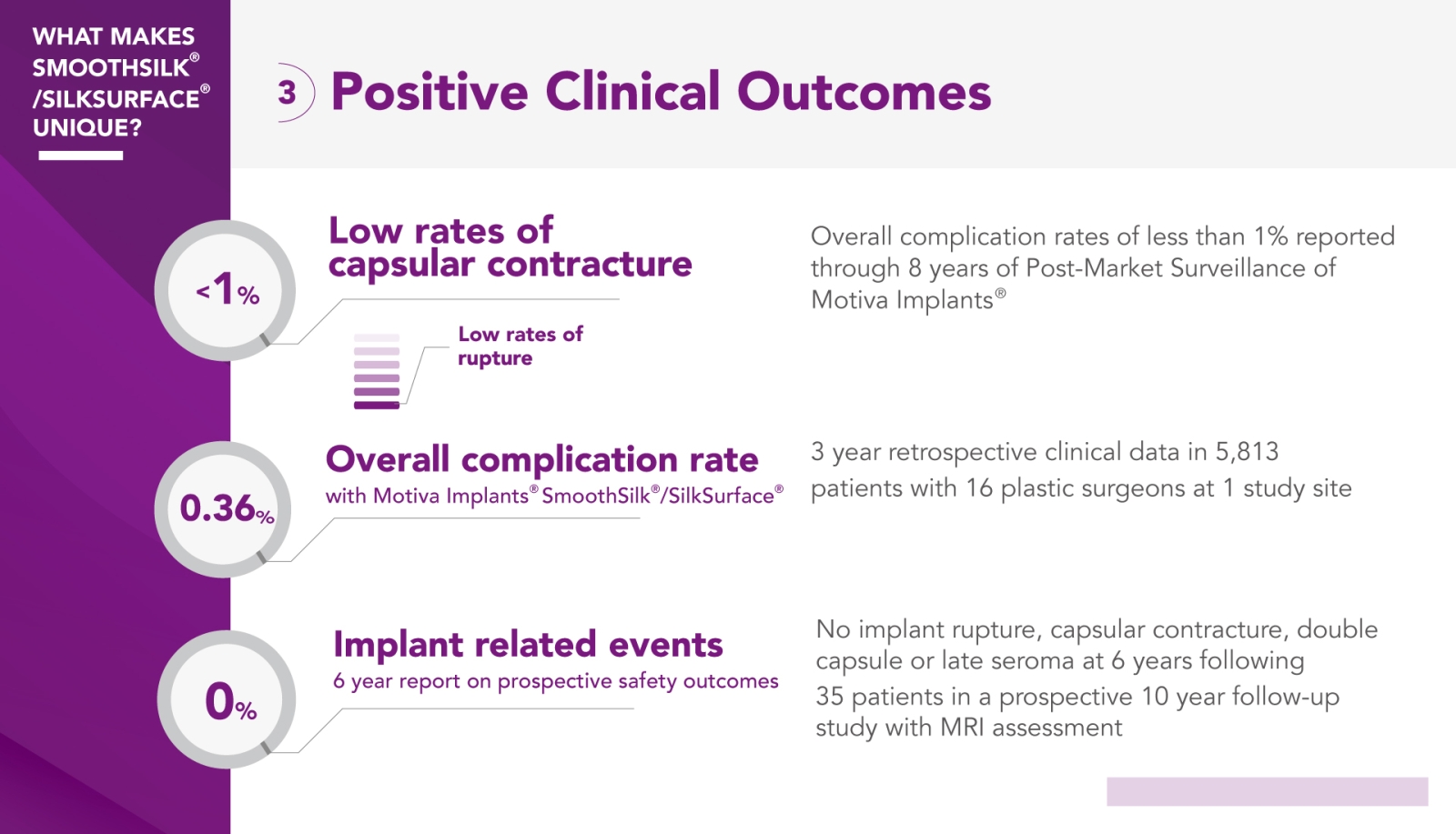
Motiva® prioritizes quality and safety and has maintained low complaint rates related to implant-related complications for over a decade.
As an added measure of confidence in the quality of our Ergonomix2® implants, the JOY® program includes the 10 Year Always Confident Warranty® and 5 Year Extended Warranty at no additional cost.
*Warranty programs are subject to the terms and conditions of the applicable warranty, which are available online, and may require timely registration for eligibility.
10Y Always Confident Warranty® against rupture for the lifetime of the device, and our Product Replacement Policy.
5Y Extended Warranty Program at no additional cost

